Introduction: Phase 1b of LUMMICAR STUDY 1 was conducted in China (NCT03975907) for zevorcabtagene autoleucel (zevor-cel, CT053), a fully human autologous chimeric antigen receptor (CAR) T-cell therapy comprising a B-cell maturation antigen-specific single-chain variable fragment, in patients with relapsed or refractory multiple myeloma (RRMM). Previously disclosed 1-year follow-up data (ASH 2021 Abstract 2821) demonstrated a tolerable safety profile and deep and durable responses in 14 patients. Here, we present the updated results with 3 years of follow-up after the last patient was infused.
Methods: Patients were eligible to be enrolled with a diagnosis of RRMM, ECOG score of 0 or 1, and they had received at least 3 prior regimens including at least one proteasome inhibitor and one immunomodulatory drug. A single infusion of zevor-cel (two dose levels, 100 × 10 6 CAR + T cells and 150 × 10 6 CAR + T cells) was administered 5-7 days after the start of lymphodepletion. Response was assessed by investigator per IMWG 2016 criteria. Bone marrow aspirates were tested for minimal residual disease (MRD) by the EuroFlow assay with a minimum sensitivity of 1 in 10 5 nucleated cells.
Results: Starting July 23, 2019, 14 patients with a median age of 54 years (range 34, 62), received a single infusion of zevor-cel (100 × 10 6 CAR + T cells in 3 patients, 150 × 10 6 CAR + T cells as the recommended phase 2 dose in 11 patients). The median number of prior lines of therapy was 6. A total of 50.0% of the infused patients had high-risk cytogenetics, 14.3% had extramedullary disease, 14.3% had ISS stage III, and no patients received bridging therapy.
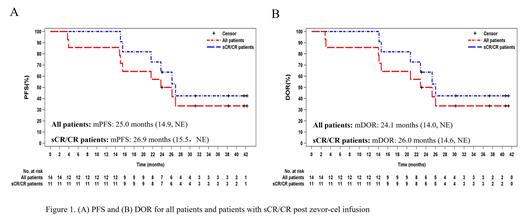
By the data cutoff date (July 17, 2023), the median survival follow-up duration was 37.7 months (range:14.8, 44.2). Overall response rate was 100% (95% CI 76.8, 100.0), in which 11 (78.6%) patients achieved complete response (CR) or stringent complete response (sCR); 2 (14.3%) patients achieved very good partial response and 1 (7.1%) patient had partial response. All patients who achieved CR or better were MRD negative. The median progression-free survival was 25.0 months (14.9, not evaluable [NE]) for all patients and 26.9 months (15.5, NE) for patients with sCR/CR (Figure 1A). The median duration of response was 24.1 months (14.0, NE) for all patients and 26.0 months (14.6, NE) (Figure 1B) for patients with sCR/CR. At data cutoff, 5 subjects still had ongoing responses, and 7 patients progressed and were still in survival follow-up. Two patients had died at month 42.6 and 32.6, respectively, and their deaths were deemed unrelated to zevor-cel.
All patients experienced treatment related adverse events and grade 3 or 4 hematologic toxicity. Thirteen patients (92.9%) had cytokine release syndrome (all grade 1 or 2). No immune effector cell-associated neurotoxicity syndrome, no second primary malignancy, and no autoimmune disease were reported. All patients have been tested negative for replication competent lentivirus to date.
Conclusions: At approximately 3 years of follow-up, heavily pre-treated RRMM patients maintained deep and durable responses after receiving a single infusion of zevor-cel, which showed a well-managed safety profile in the ongoing long-term follow-up.
Disclosures
Meng:CARsgen Therapeutics Co. Ltd: Current Employment. Zheng:CARsgen Therapeutics Co. Ltd: Current Employment. Wang:CARsgen Therapeutics Co. Ltd: Current Employment. Li:CARsgen Therapeutics Co. Ltd: Current Employment.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal